Is your daily routine toxic?
Because of a lack of regulation, many cosmetics and personal care products contain potentially toxic ingredients, like formaldehyde and lead acetate. What toxic chemicals might you encounter as you go about your daily routine?
Sodium laureth sulfate, PPG-5-ceteth-20, and fragrance. Those are just a few of the tongue-twisting and toxic ingredients in one Garnier Fructis shampoo — and many other cosmetic products.[1]
For years, we’ve dealt with a system that fails to protect us from toxic chemicals in our products. The average person is exposed to more than 100 chemicals from personal care products – many of them harmful – before leaving the house in the morning.[2]
Why? Because the Food and Drug Administration (FDA) doesn’t have the power to test products for safety before they hit the market – in fact, the FDA can’t even recall a dangerous product.[3] That’s how chemicals linked to cancer, reproductive harm, and other health problems — like formaldehyde and lead acetate — end up in our cosmetic goods. And that’s also why manufacturers have not only the power — but a responsibility — to make their products safer. We’re asking Procter & Gamble, Unilever, and L’Oreal, the manufacturing giants behind popular brands like Old Spice, Olay, Axe, Degree, Dove, and Garnier, to go toxic-free. Join our call now.
What toxic chemicals might you encounter as you go about your daily routine? Find out below, and check out our consumer guide on which products to avoid here.






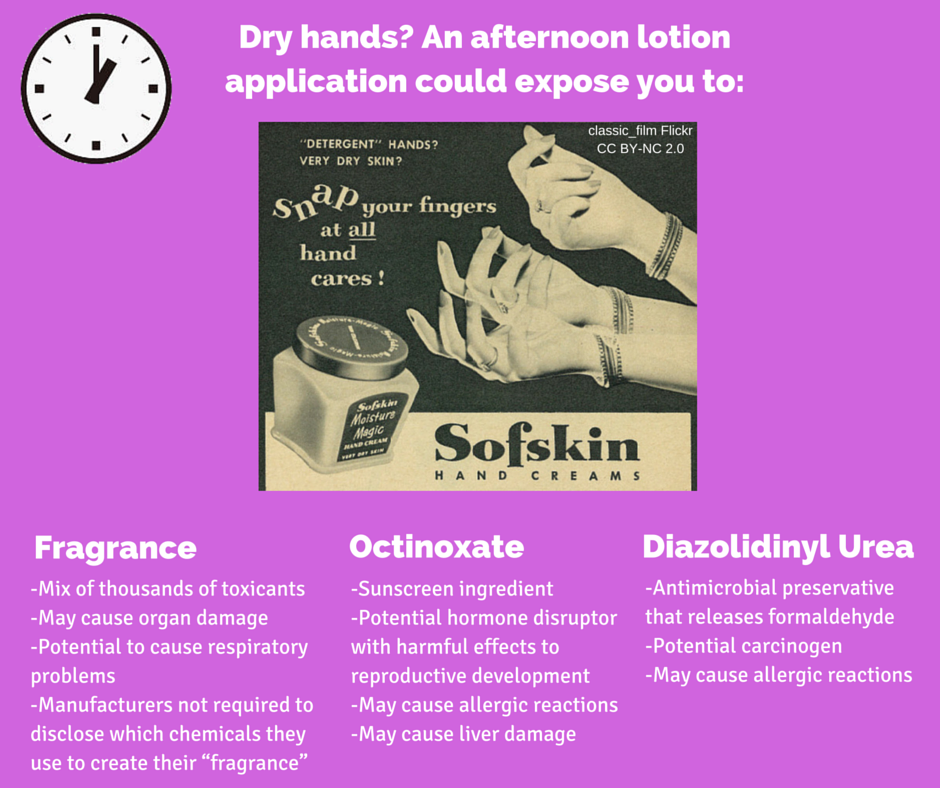

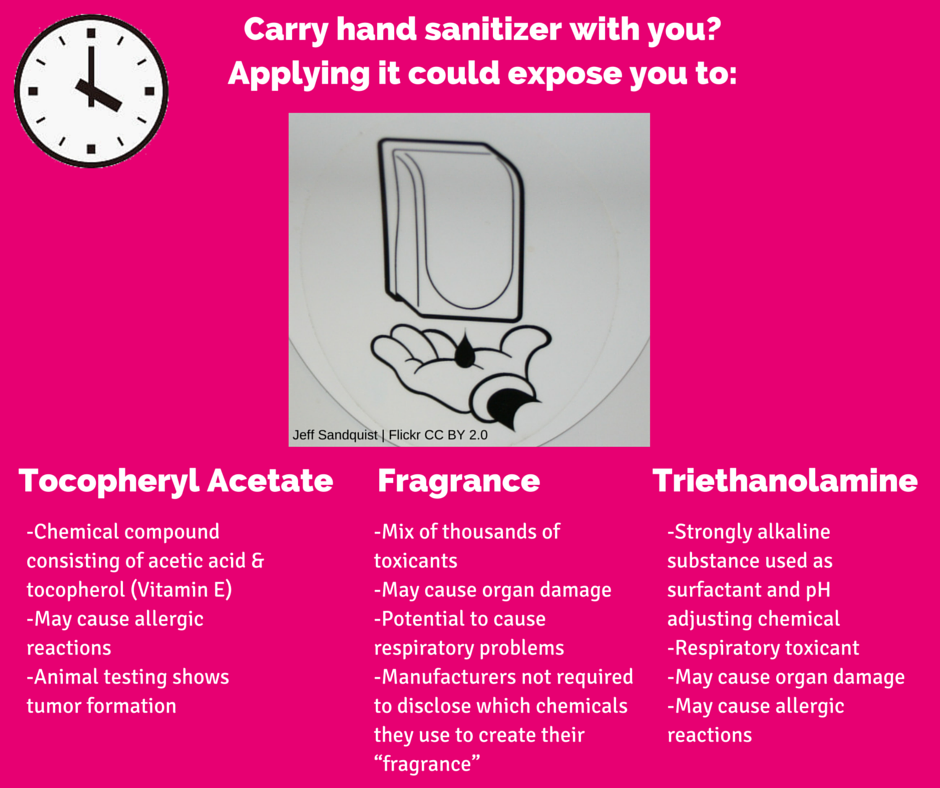


[1] Garnier, “Garnier Fructis Damage Eraser Shampoo,” Viewed July 2016.
[2] Harvard T.H. Chan School of Public Health, “Harmful, untested chemicals rife in personal care products,” February 13, 2014.
[3] Food and Drug Administration, “FDA Authority Over Cosmetics,” August 3, 2013.
Sources:
European Commission. 2013. “Cosing, the European Commission database with information on cosmetic substances and ingredients,” Viewed 12/4/15.
National Institute of Environmental Health, “Butylparaben: Review of Toxicological Literature,” Viewed 12/4/15.
Charles AK, Darbre PD. 2009. “Oestrogenic activity of benzyl salicylate, benzyl benzoate and butylphenylmethylpropional (Lilial) in MCF7 human breast cancer cells in vitro,” J Appl Toxicol. 29(5): 422-34. Viewed 12/4/15.
Kumar P, Caradonna-Graham VM, Gupta S, Cai X, Rao PN, Thompson J. 1995. “Inhalation challenge effects of perfume scent strips in patients with asthma,” Ann Allergy Asthma Immunol. 75(5): 429-33. Viewed 12/4/15.
Dodson, RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Ruthann AR, “Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products,” Viewed 12/4/15.
Scientific Committee on Consumer Safety. “OPINION on Fragrance allergens in cosmetic products,” Viewed 12/4/15.
California Environmental Protection Agency, “Office of Environmental Health Hazard Assessment, Safe Drinking Water and Toxic Enforcement Act of 1986, Chemicals known to the State to cause cancer or reproductive toxicity,” Viewed 12/4/15.
International Agency for Research on Cancer, “Coconut Oil Diethanolamine Condensate,” Viewed 12/4/15.
Du S, McLaughlin BA, Pal S, Aizenman E. 2002. “In Vitro Neurotoxicity of Methylisothiazolinone, a Commonly Used Industrial and Household Biocide, Proceeds via a Zinc and Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase-Dependent Pathway,” The Journal of Neuroscience 22(17): 7408-7416. Viewed 12/4/15.
Cosmetic Ingredient Review, “Amended Safety Assessment of Methylisothiazolinone, as Used in Cosmetics,” Viewed 12/4/15.
Hanson KM, Gratton E, Bardeen CJ. 2006. “Sunscreen enhancement of UV-induced reactive oxygen species in the skin,” Free Radic Biol Med 41(8): 1205-1212. Viewed 12/5/15.
Klammer H, Schlecht C, Wuttke W, Schmutzler C, Gotthardt I, Kohrle J, Jarry H. “Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamopituitary-thyroid function in rats,” Toxicology 238(2-3): 192-9. Viewed 12/4/15.
Rodriguez E, Valbuena MC, Rey M, Porras de Quintana L. 2006. “Causal agents of photoallergic contact dermatitis diagnosed in the national institute of dermatology of Colombia,” Photodermatol Photoimmunol Photomed 22(4): 189-192. Viewed 12/4/15.
EC (Environment Canada). 2008. Domestic Substances List Categorization. Canadian Environmental Protection Act (CEPA) Environmental Registry.
Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. 2006. “The bacterial agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development,” Aquat Toxicol. 80(3): 217-27. Viewed 12/4/15.
Cherednichenko G, Zhang R, Bannister RA, Timofeyev V, Li N, Fritsch EB, et al. 2012. “Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle,” Proceedings of the National Academy of Sciences of the United States of America 109(35): 14158-14163. Viewed 12/4/15.
U.S. Environmental Protection Agency, “Hydroquinone: Hazard Summary,” Viewed 12/4/15.
Hathcock JN, Hattan DG, Jenkens MY, McDonald JT, Sundaresan PR, Wilkening VL. 1990. “Evaluation of vitamin A toxicity,” Am J Clin Nutr 52: 183-202. Viewed 12/4/15.
Halliday GM, Robertson BO, Barnetson RS. 2000. “Topical retinoic acid enhances, and a dark tan protects, from subedemal solar-simulated photocarcinogenesis,” J Invest Dermatol 114(5): 923-7. Viewed 12/4/15.
Scientific Committee on Consumer Safety (SCCS), 2011. “OPINION ON Quaternium-15 (cis-isomer), COLIPA n P63,” Viewed 12/4/15.
PubChem Open Chemistry Database, “Quaternium-15,” Viewed 12/4/15.
European Food Safety Authority. 2012. EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS); Scientific Opinion on the re-evaluation of Butylated hydroxytoluene BHT (E 321) as a food additive. EFSA Journal 10(3), 2588.
Harris G Vikis, Alexander S. Krupnick, Jay W. Tichelaar, Pengyuan Liu, Jihong Zhu, Amy Rymaszewski, Lauren Stein, Andrew Franklin, Andrew E. Gelman & Ming You. 2011. “Neutrophils are required for 3-methylcholanthrene-initiated, butylated hydroxytoluene-promoted lung carcinogenesis.” Molecular carcinogenesis.
Janjua NR, Mogensen B, Andersson AM, Petersen JH, Henriksen M, Skakkebaek NE, et al. 2004. “Systemic Absorption of the Sunscreen Benzophenone-3, Octyl-Methoxycinnamate, and 3-(4-Methyl-Benzylidene) Camphor after Whole-Body Topical Application and Reproductive Hormone Levels in Humans,” Journal of Investigative Dermatology 123(1): 57-61.
Schneider. 2005. “Octyl methoxycinnamate: two generation reproduction toxicity in Wistar rats by dietary administration.” Food Chem Toxicol. 43(7): 1083-92.
IARC (International Agency for Research on Cancer). 2008. “Overall Evaluations of Carcinogenity to Humans, as evaluated in IARC Monographs Volumes 1-99,” Viewed 12/7/15.
De Groot AC, Whie IR, Flyvholm MA, Lensen G, Coenraads PJ. 2010. “Formaldehyde-releasers in cosmetics: relationship to formaledhyde contact allergy,” Contact Dermatitis. Viewed 12/7/15.
Yourick JJ, Yung CT, Bronaugh RL. 2008. “In vitro and in vivo percutaneous absorption of retinol from cosmetic formulations: significance of the skin reservoir and prediction of systemic absorption,” Toxicol. Appl. Pharmacol. Viewed 12/7/15.
Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, et al. 1995. “Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acide levels or irritation,” J Invest Dermatol 105(4): 549-56. Viewed 12/7/15.
Cosmetic Ingredient Review, “Safety Assessment of Tocopherols and Tocotrienols as Used in Cosmetics,” Viewed 12/7/15.
AOEC (Association of Occupational and Environmental Clinics). 2009. AEOC exposures codes and asthmagen designation.
Byford JR, Shaw LE, Drew MG, Pope GS, Sauer MJ, Darbre PD. “Oestrogenic activity of parabens in MCF7 human breast cancer cells,” J Steroid Biochem Mol Biol. 80(1): 49-60. Viewed 12/7/15.
EPA (U.S. Environmental Protection Agency). 1999. Toxics Release Inventory Program. PBT Chemical Rule.
Cherng SH, Xia Q, Blankenship LR, Freeman JP, Wamer WG, Howard PC, et al. 2005. “Photodecomposition of retinyl palmitate in ethanol by UVA light-formation of photodecomposition products, reactive oxygen species, and lipid peroxides,” Chem Res Toxicol 18(2): 129-38. Viewed 12/7/15.
Boberg J, Taxvig C, Christiansen S, Hass U. 2010. “Possible endocrine disrupting effects of parabens and their metabolites,” Reprod Toxicol. 30(2): 301-12. Epub 2010 Apr. 8. Viewed 12/7/15.
